BA/BE
Bioavailability (BA) : Bioavailability refers to the relative amount of drug from an administered dosage from which enters the systemic circulation and the rate at which the drug appears in the systemic circulation.
Bioequivalence (BE): Bioequivalence of a drug product is achieved if its extent and rate of absorption are not statistically significantly different from those of the reference product when administered at the same molar dose.
The office of Drugs Controller General (India) at CDSCO (HQ) FDA Bhawan, New Delhi has been receiving applications from applicants requesting for the approval to carry out BA/BE studies with various pharmaceutical dosage formulations on Indian subjects. The office of DCG(I) would like to ensure the demonstration of the safety and tolerability of generics against corresponding innovator drugs; to ensure they are comparable and safe for consumption by human subjects. An Assessment of "interchangeability" between the investigational and the innovator product is carried out by a study of "in vivo equivalence" or "bioequivalence" (BE). In view of the above, the office of DCGI would like to ensure the uniformity of documents to be submitted to this Directorate for review and approval of BE-NOC for export to meet tenets of Schedule Y of Drugs and Cosmetics Act 1940 & Rules 1945 and also Indian Good Clinical Practices (GCP) guidelines.
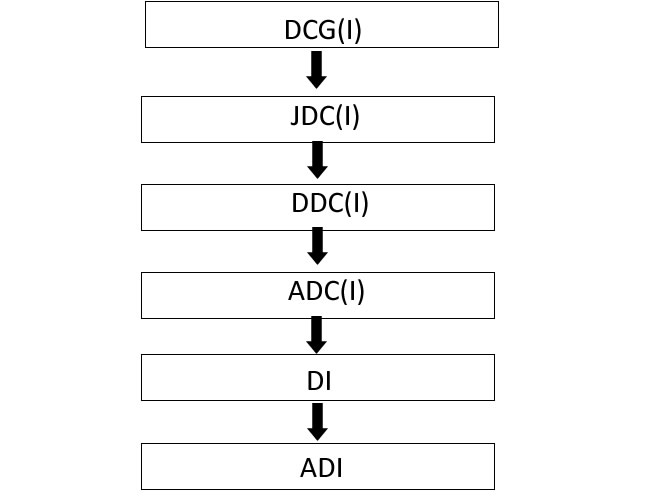
The applicants submit the application for BE NOC & Test Licence through online Sugam Portal in applicable checklist. The application will be processed by the Assistant Drugs Inspector/Drugs Inspector (Reviewing Officer) and send to the ADC(I) (Nodal Officer) for further review. The Nodal Officer will send the application to DDC(I) (Deputy Decision Authority)/JDC(I) (Decision Authority) for recommendation for approval then DCG(I) (Licensing Authority) will grant the BE NOC & Test Licence to the applicant through Online Sugam Portal.