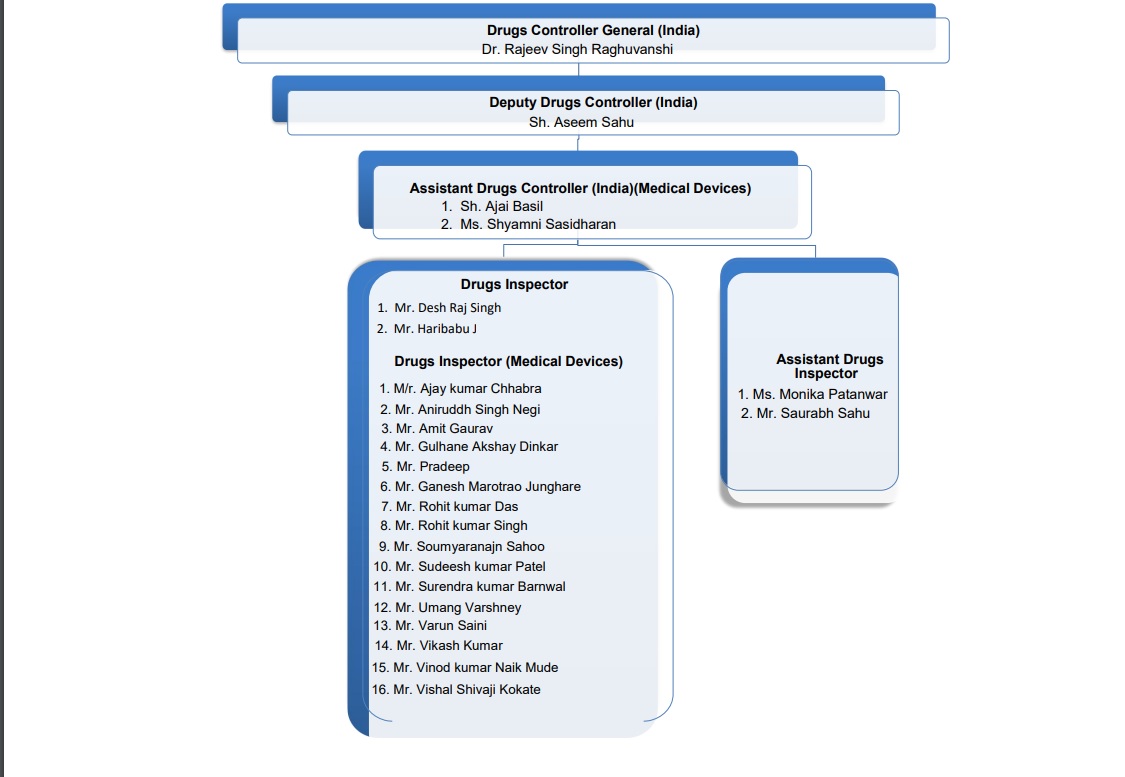
| 1 |
Notice to All stakeholders for Strengthening of Private Medical Device Testing Laboratory |
2024-Apr-03 |
|
388 KB |
| 2 |
Online application for Neutral Code for manufacturing of Medical Devices for export purpose |
2024-Feb-12 |
|
305 KB |
| 3 |
Launching of National Single Window System (NSWS) Portal |
2024-Jan-01 |
|
3669 KB |
| 4 |
Clarification on the Circular no. 29/Misc/03/2023-DC(344) dated 12.10.2023 |
2023-Oct-12 |
|
22 KB |
| 5 |
Regulation of all Class C & D Medical Devices Under Licensing regime, w.e,f 01.10.2023 as per GSR102E dated 11.02.2020 |
2023-Oct-12 |
|
385 KB |
| 6 |
List of medical devices testing laboratory (MDTL) for carry out test or evaluation of medical device on behalf of manufacturer registered with CDSCO under MDR 2017 |
2023-Sep-15 |
|
1635 KB |
| 7 |
Circular for Licensing of Class C D medical devices dated 12.04.2023 |
2023-Apr-12 |
|
310 KB |
| 8 |
List of Notified Bodies registered with CDSCO under MDR, 2017 |
2023-Apr-03 |
|
830 KB |
| 9 |
Registration of Medical Device Testing Laboratory in Form MD-40 as per Medical Devices Rules (MDR) 2017 for Testing of Medical Devices on behalf of the manufacturer |
2022-Dec-22 |
|
412 KB |
| 10 |
Classification of Medical Device pertaining to Oncology under the provisions of Medical Devices Rules, 2017 |
2022-Oct-11 |
|
817 KB |
| 11 |
Classification of Medical Device Pertaining to Dental Under the provision of Medical Devices Rules 2017 |
2022-Oct-10 |
|
764 KB |
| 12 |
Regulation of all Class A & B Medical Devices under Licensing regime, w.e.f 01.10.2022, as per G.S.R. 102(E) dt 11.02.2020 |
2022-Sep-30 |
|
401 KB |
| 13 |
Notice regarding implementation of gazette notification vide GSR 754(E) dated 30.09.2022 |
2022-Sep-30 |
|
290 KB |
| 14 |
Strengthening of Medical Products Safety Surveillance System in North Eastern States of India on October 19 2022 at Government Pharmacy College Sajong Sikkim |
2022-Sep-29 |
|
1476 KB |
| 15 |
Classification of Medical Device Pertaining to non-sterile, non-powered, hand-held or hand-manipulated Surgical Instruments for general use intended to be used in various general surgical procedures |
2022-Sep-09 |
|
524 KB |
| 16 |
List of the Certified Medical Device Testing Laboratory under MDR, 2017 |
2022-Aug-17 |
|
5938 KB |
| 17 |
Classification of Medical Device Pertaining to Rehabilitation under the Provisions of Medical Devcies Rules 2017 |
2022-Aug-04 |
|
852 KB |
| 18 |
Notice dated July 11 2022 |
2022-Jul-11 |
|
370 KB |
| 19 |
Classification of Medical Device Pertaining to Obstetrical and Gynecological Under the provision of Medical Devices Rules 2017 |
2022-Jun-03 |
|
529 KB |
| 20 |
Classification of Medical Device pertaining to General Hospital under the provisions of Medical Devices Rules 2017 |
2022-Mar-16 |
|
813 KB |
| 21 |
Regulation of CT scan equipment_All Implantable Devices_MRI equipment etc. as Drugs with effect from April 1st 2021 |
2021-Nov-03 |
|
246 KB |
| 22 |
Registration and Labelling requirements of Medical Devices |
2021-Sep-28 |
|
366 KB |
| 23 |
Classification of Medical Device pertaining to Neurological under the provisions of Medical Devices Rules, 2017 |
2021-Sep-28 |
|
367 KB |
| 24 |
Classification of Medical Device pertaining to Gastroenterology under the provisions of Medical Devices Rules, 2017 |
2021-Sep-27 |
|
929 KB |
| 25 |
Guidance Document for Manufacturers / Importers for voluntary/ mandatory registration of Medical Devices |
2021-Sep-21 |
|
513 KB |
| 26 |
Classification of Medical Device pertaining to Personal Protective Equipment under the provisions of Medical Devices Rules 2017 |
2021-Sep-13 |
|
674 KB |
| 27 |
Classification of Medical Device pertaining to Operation Theatre under the provisions of Medical Devices Rules 2017 |
2021-Sep-13 |
|
804 KB |
| 28 |
Classification of Medical Device pertaining to Nephrology and Renal Care under the provisions of Medical Devices Rules 2017 |
2021-Sep-13 |
|
744 KB |
| 29 |
Classification of Medical Device pertaining to Pain Management under the provisions of Medical Devices Rules 2017 |
2021-Sep-13 |
|
334 KB |
| 30 |
Classification of Medical Device pertaining to Software under the provisions of Medical Devices Rules 2017 |
2021-Sep-13 |
|
698 KB |
| 31 |
The list of standards published under Medical Equipment and Hospital Planning (MHD) of BIS as forwarded by BIS for ready reference |
2021-Sep-07 |
|
665 KB |
| 32 |
List of Medical devices testing Laboratory for carry out test or evaluation of Medical device on behalf of Manufacturer registered with CDSCO under MDR |
2021-Aug-27 |
|
3982 KB |
| 33 |
Classification of Medical Device Pertaining to Pediatrics and Neonatology Under the provision of Medical Devices Rules 2017 |
2021-Aug-23 |
|
1120 KB |
| 34 |
Classification of Medical Device Pertaining to Urology Under the provision of Medical Devices Rules 2017 |
2021-Aug-23 |
|
955 KB |
| 35 |
Classification of Medical Devices Pertaining to Ophthalmology under the Provisions of Medical Devices Rules 2017 |
2021-Aug-09 |
|
889 KB |
| 36 |
Classification of Medical Device Pertaining to Radiotherapy under the Provisions of Medical Devcies Rules 2017 dated 6 August 2021 |
2021-Aug-06 |
|
606 KB |
| 37 |
Classification of Medical Devices Pertaining to ENT under the Provisions of Medical Devcies Rules 2017 dated 6 August 2021 |
2021-Aug-06 |
|
601 KB |
| 38 |
Classification of Medical Device Pertaining to Respiratory Under the Provisions of Medical Devcies Rules 2017 dated 6 August 2021 |
2021-Aug-06 |
|
628 KB |
| 39 |
Classification of medical device pertaining to Cardiovascular under the provisions of Medical Devices Rules 2017 |
2021-Jul-26 |
|
523 KB |
| 40 |
Classification of Medical Device Pertaining to Interventional Radiology under the Provisions of Medical Devcies Rules 2017 |
2021-Jul-26 |
|
432 KB |
| 41 |
Classification of Medical Device Pertaining to Dermatological and Plastic Surgery under the Provisions of Medical Devcies Rules 2017 |
2021-Jul-26 |
|
256 kB |
| 42 |
Classification of Medical Device Pertaining to Physical Support under the Provisions of Medical Devcies Rules 2017 |
2021-Jul-26 |
|
618 KB |
| 43 |
Classification of medical devices pertaining of Anesthesiology under the provision of Medical Devices Rules 2017 |
2021-Jul-12 |
|
200 KB |
| 44 |
List of medical devices testing laboratory for carry out test or evaluation of medical device on behalf of manufacturer with CDSCO under MDR 2017 |
2021-Jul-07 |
|
4025 KB |
| 45 |
Regulation of CT Scan equipment, All Implantable Devices, MRI equipment etc. as Drugs with effect from April 1st 2021 |
2021-Apr-18 |
|
138 KB |
| 46 |
Regulation of Blood Glucose monitors Blood pressure monitors Nebulizers and Thermometers as drugs with effect from Jan 1st 2021 |
2020-Dec-28 |
|
334 KB |
| 47 |
Notice regarding List of medical devices testing Laboratory |
2020-Oct-09 |
|
2927 KB |
| 48 |
Online Application for Free Sale certificate Market standing certificate and Non-conviction certificate of Notified Medical Devices |
2020-Sep-03 |
|
301 KB |
| 49 |
Notice regarding classification of non notified medical devices1 |
2020-Sep-03 |
|
12557 KB |
| 50 |
Requirement of fees for change in address of authorized agent without change in constitution as post approval Change under MDR 2017 |
2020-Aug-31 |
|
362 KB |
| 51 |
Submission of notarized/apostilled documents for import of medical device and in-vitro diagnostic kits in view of Covid-19 |
2020-Aug-31 |
|
343 KB |
| 52 |
Clarification regarding import of diagnostic kits/ reagents for Research use only ROU for academic research purpose |
2020-Jun-19 |
|
368 KB |
| 53 |
Submission of notarized/apostilled document for import and registration of medical devices in view of covid-19 |
2020-Apr-23 |
|
307 KB |
| 54 |
Strengthening of Materiovigilance Programme of India (MvPI) in the country |
2019-Nov-26 |
|
56 KB |
| 55 |
Processing of application for product approved as medical device in the country of origin, but covered under the definition of drug in the country-reg |
2019-Nov-15 |
|
289 KB |
| 56 |
Notice regarding equipments which are non notified but associated with notified devices |
2019-Nov-13 |
|
226 KB |
| 57 |
Notice regarding environmental conditions for equipments |
2019-Nov-08 |
|
235 KB |
| 58 |
Notice regarding all implantable devices |
2019-Nov-08 |
|
252 KB |
| 59 |
Notice regarding exemption of drugs Sale License |
2019-Nov-08 |
|
235 KB |
| 60 |
Notice regarding Notification to notify all the medical device under sub-section (b) of Section 3 of th Drugs and Cosmetics Act 1940 to regulate them as per the provisions of the Act and Medical Devices Rules 2017 |
2019-Oct-18 |
|
1962 KB |
| 61 |
Constitution of Medical Devices Technical advisory group (MDTAG) to advise CDSCO on matter related to regulation of medical devices-Reg |
2019-Jul-22 |
|
650 KB |
| 62 |
Classification of Newly Notified Medical Devices to be updated with Classification list of Medical Devices and IVDs |
2019-May-15 |
|
2021 KB |
| 63 |
Formula for grant of Compensation to the patients who were implanted with ASR Hip and Constitution of the state Level Committee |
2018-Nov-30 |
|
2586 KB |
| 64 |
Health Ministry approves compensation formula for Hip implant cases |
2018-Nov-29 |
|
157 KB |
| 65 |
Application form for Compensation-DePuy_Johnson |
2018-Nov-15 |
|
118 KB |
| 66 |
To provide guidance and advertisement for compensation to patients implanted with ASR Hip Implants |
2018-Nov-08 |
|
439 KB |
| 67 |
Advertisement of ADI |
2018-Sep-30 |
|
425 KB |
| 68 |
Constitution of State level Committee Regarding the Faulty ASR HIP Implant manufatured by M/s DePuy International Ltd U K |
2018-Sep-28 |
|
352 KB |
| 69 |
Office Order |
2018-May-11 |
|
265KB |
| 70 |
Office Order |
2018-May-01 |
|
245KB |
| 71 |
Office Order regarding Public Relations Office at CDSCO (HQ) |
2018-Apr-23 |
|
245KB |
| 72 |
Grouping Guidelines for Medical Devices Applications |
2018-Mar-16 |
|
1,984kb |
| 73 |
Office Order regarding Setting up of Public Relation Office at CDSCO (HQ) dated 07.3.2018 |
2018-Mar-07 |
|
310KB |
| 74 |
Notice regarding Finalization of audit fee of Notified Bodies under Medical Devices Rules,2017 |
2018-Feb-21 |
|
9728KB |
| 75 |
Office Corrigendum regarding Drugs Inspector's transfers in CDSCO |
2018-Feb-20 |
|
204KB |
| 76 |
Order regarding Drugs Inspector's/Technical officer's transfers |
2018-Feb-20 |
|
931KB |
| 77 |
Report of the Expert Committee to address the Issue of Faulty ASR⢠Hip Implants (Dr. Arun Kumar Aggarwal, The Chairman of the Expert Committee) |
2018-Feb-19 |
|
34851 KB |
| 78 |
Office order regarding Grievance Redressal in CDSCO (HQ) |
2018-Feb-13 |
|
304KB |
| 79 |
Notice regarding application were called for engagement of Research Scientist medical Device and Diagnostic on contractual basis under CDSCO H/Q |
2018-Jan-23 |
|
606KB |
| 80 |
Office Memorandum regarding Filling up the post of DIs(Medical Devices) in CDSCO on deputation basis |
2018-Jan-22 |
|
823KB |
| 81 |
Office order regarding appointed to offciate on ad-hoc basis on the post of ADC(I) |
2018-Jan-22 |
|
875KB |
| 82 |
NOTICE - regarding Draft guidance documents on essential principles for safety and performance of medical devices as per medical devices rules 2017 |
2018-Jan-01 |
|
1.16MB |
| 83 |
Office order regarding SUGAM Portal of CDSCO |
2017-Dec-11 |
|
180KB |
| 84 |
Office order regarding appointment of CPIO and Applellate Authority u/s 5 of RTI 2005 for RDTL Chandigarh |
2017-Dec-05 |
|
308kb |
| 85 |
Notice Regarding Classification of Medical devices and in Vitro diagnostic Medical devices under the provisions of the Medical Devices Rules 2017 |
2017-Nov-01 |
|
4,147 KB |
| 86 |
Office Memorandum regarding clarification for dealing with impoted consignments of Medical Devices and IVDs held at the port offices |
2017-Oct-31 |
|
665KB |
| 87 |
NOTICE regarding Not of Standard Quality Combipack ABD |
2017-Sep-15 |
|
72.8KB |
| 88 |
NOTICE regarding not of Standard Quality of Mediclone D |
2017-Sep-15 |
|
59.9KB |
| 89 |
NOTICE - regarding Setting up of GST helpdesk in CDSCO (HQ) |
2017-Jul-17 |
|
182KB |
| 90 |
NOTICE - regarding availability of essential mediines following implementation of GST |
2017-Jul-13 |
|
637KB |
| 91 |
corrigendum with respect to revised specification/criteria of acceptance for quality test for Ant-HCV Rapid Kit |
2017-Jul-12 |
|
41.9KB |
| 92 |
Notice Order regarding draft classification of Medical Devices and IVDs |
2017-Jun-29 |
|
1.83MB |
| 93 |
Notice Order regarding Streamlining the regulatory procedures |
2017-Jun-27 |
|
212kb |
| 94 |
Notice Order regarding address the grievances/ appeals |
2017-Jun-22 |
|
642kb |
| 95 |
Notice Order regarding Oseltamivir and Zanamivir |
2017-Jun-22 |
|
1.14MB |
| 96 |
Notice regarding Draft SOP for handling of NSQ samples |
2017-Jun-13 |
|
5.57KB |
| 97 |
Notice regarding Online application for registration of Notified Body through SUGAM Portal under medical Devices Rules 2017 |
2017-May-30 |
|
279KB |
| 98 |
Office Memorandum permission to affix the sticker indicating date of manufacture and Date of expiry with respect of Medical Devices under the provision of Drugs and Cosmetic Rules |
2017-May-04 |
|
60.1KB |
| 99 |
NOTICE - regarding testing of Notified Diagnostics kits for hepatiitis Kit at ILBS New Delhi |
2017-Apr-24 |
|
37.5KB |
| 100 |
Notice regarding Meeting for the registration of notified body through online portal |
2017-Apr-07 |
|
542KB |
| 101 |
Notice regarding Issue of Updating the Schedule H of the Drugs and Cosmetics Rules 1945 |
2017-Mar-31 |
|
250KB |
| 102 |
Public Notice regarding Strict regulatory control over manufacture, sale and distribution of oxytocin and to curb its misuse |
2017-Mar-30 |
|
213KB |
| 103 |
Office memorandum regarding Strict regulatory control over manufacture, sale and distribution of oxytocin and to curb its misuse |
2017-Mar-30 |
|
287KB |
| 104 |
Notice regarding Regulatory approvals relating to combination products for HIV Hepatitis B&C which are recommended for Concomitant use by WHO |
2017-Mar-20 |
|
432KB |
| 105 |
Notice regarding Streamline the grievance |
2017-Feb-02 |
|
362KB |
| 106 |
Notice regarding Medical Devices Rules,2017 Notification No. 78E |
2017-Feb-02 |
|
23.0KB |
| 107 |
List of clarifications and NOC issued from 2011 to 2017 |
2017-Jan-02 |
|
901kb |
| 108 |
Extending the validity of Free Sale certificate for notified medical devices |
2016-Apr-21 |
|
650KB |
| 109 |
Notice regarding Launching of Online portal SUGAm for import and registration of Medical Devices and Diagnostics |
2016-Mar-04 |
|
673KB |
| 110 |
Acceptance Criteria for test and Examination of Blood Glucose Test Trips and Analyzer based glucose reagen |
2016-Feb-16 |
|
54KB |
| 111 |
Clarification for import of in Bulk form in Sutures |
2015-Dec-08 |
|
258kb |
| 112 |
Latter to All state/UT Drugs Controllers regarding Availabilty of diagnostic Kits, Drugs used for swine Flue |
2015-Feb-14 |
|
294KB |
| 113 |
Office Order dated 09.07.2014 Clarification for regulation of Medical devices under the provision of Drugs and Cosmetics Acts and Rules |
2014-Jul-09 |
|
437KB |
| 114 |
Clarification reg NOC to import of diagnostics kit or reagent for ROU dated 13.07.2012 |
2012-Jul-13 |
|
201 KB |
| 115 |
Regulation of import and manufacture of certain medical devices covered under the Categories of notified Medical devices |
2012-Mar-05 |
|
74 KB |
| 116 |
ORTHOPAEDIC ORDER |
2012-Feb-17 |
|
127 KB |
| 117 |
GENERAL ORDER |
2012-Feb-17 |
|
140 KB |
| 118 |
REPRODUCTIVE & UROLOGY ORDER |
2012-Feb-17 |
|
123 KB |
| 119 |
MISCELLANEOUS ORDER |
2012-Feb-17 |
|
118 KB |
| 120 |
Clarification for import of pre-filled syringe |
2011-Nov-07 |
|
282kb |
| 121 |
Clarification on regulation of staplers |
2011-Oct-04 |
|
54kb |
| 122 |
MDAC of Reporductive and Urology |
2011-May-10 |
|
123 KB |
| 123 |
MDAC of Orthopedic |
2011-May-10 |
|
127 KB |
| 124 |
MDAC of Ophthalmic |
2011-May-10 |
|
126 KB |
| 125 |
MDAC of General Expert Pool |
2011-May-10 |
|
140 KB |
| 126 |
MDAC Miscellaneous Devices |
2011-May-10 |
|
118 KB |
| 127 |
MDAC Dental |
2011-May-10 |
|
119 KB |
| 128 |
MDAC Cardiovascular order dated 10 May 2011 |
2011-May-10 |
|
129 KB |
| 129 |
OPHTHALMIC ORDER |
2011-Feb-17 |
|
126 KB |
| 130 |
CARDIOVASCULAR ORDER |
2011-Feb-17 |
|
129 KB |
| 131 |
DENTAL ORDER |
2011-Feb-17 |
|
119 KB |
| 132 |
List of Notified Medical devices |
2010-Apr-20 |
|
85 KB |
 Guidelines Document
Guidelines Document
 Sugam User Manual
Sugam User Manual FAQ
FAQ
 Checklist Documents
Checklist Documents