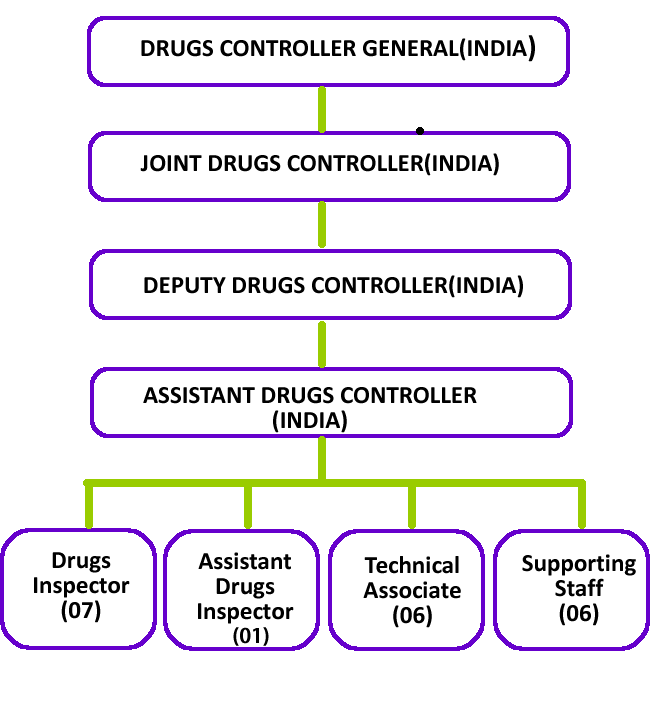
Serious Adverse Event
-
Processing of reports of SAE cases (i.e. Appendix-XI), review, approval for payment of compensation in cases of related cases as per Schedule Y, Rule 122 DAB under Drugs & Cosmetics Rules 1945)
-
Handling of Parliament questions and RTI queries related to SAEs.
-
Conduct of GCP Inspections based on the documents / information submitted for reporting of SAEs etc.
-
Review & action taken in respect to GCP inspection reports (Show-cause notice, response of show-cause notice, warning, debarment, suspension etc.)
-
Data-base management (receipts/files/other misc. data including Related and not related orders, maintenance of guard files, parliament questions answered etc.)
Currently there is no data is available.